Abstract
Introduction
Allogeneic hematopoietic cell transplantation (allo-HCT) is a potentially curative treatment option in patients with advanced Chronic Myeloid Leukemia (CML) who have disease resistant to tyrosine kinase inhibitors (TKI) or who cannot tolerate these agents. In the absence of a matched sibling donor (MSD), the use of an unrelated donor (UD) is recommended; however, this approach is associated with a higher incidence of both graft-versus-host disease (GvHD) and non-relapse mortality (NRM). Post-transplant cyclophosphamide (PTCy) has been found to result in similar rates of GvHD-free, relapse-free survival (GRFS) in allo-HCT using UD, haploidentical donors (HD) and MSD across a range of haematological malignancies. However, the role of PTCy in allo-HCT using UD and HD in CML is unknown. We here report an observational, retrospective, comparative study on the role of PTCy in UD and HD in CML patients undergoing allo-HCT.
Methods
Inclusion criteria were age ≥ 18 years, diagnosis of CML, first AlloHCT from 2012 to 2019, and UD and HD. The main endpoints of the study were to compare the one-year GRFS of allo-HCT using UD with standard GvHD prophylaxis (SP), PTCy UD and PTCy HD. Secondary endpoints were 3-year OS, 3-year PFS, 3-year NRM, 3-year RI, Day +100 acute GvHD (aGvHD) and one-year chronic GvHD (cGvHD). The Kaplan-Meier estimator and log-rank test were used for GRFS, OS and PFS, and the crude cumulative incidence estimator and Gray's test were used for competing events (RI/NRM, aGvHD/death before aGvHD and cGvHD/death before cGvHD). Cox proportional hazard regression was used in multivariable analyses (MVA) using patients with complete data with cause-specific models for competing risk outcomes.
Results
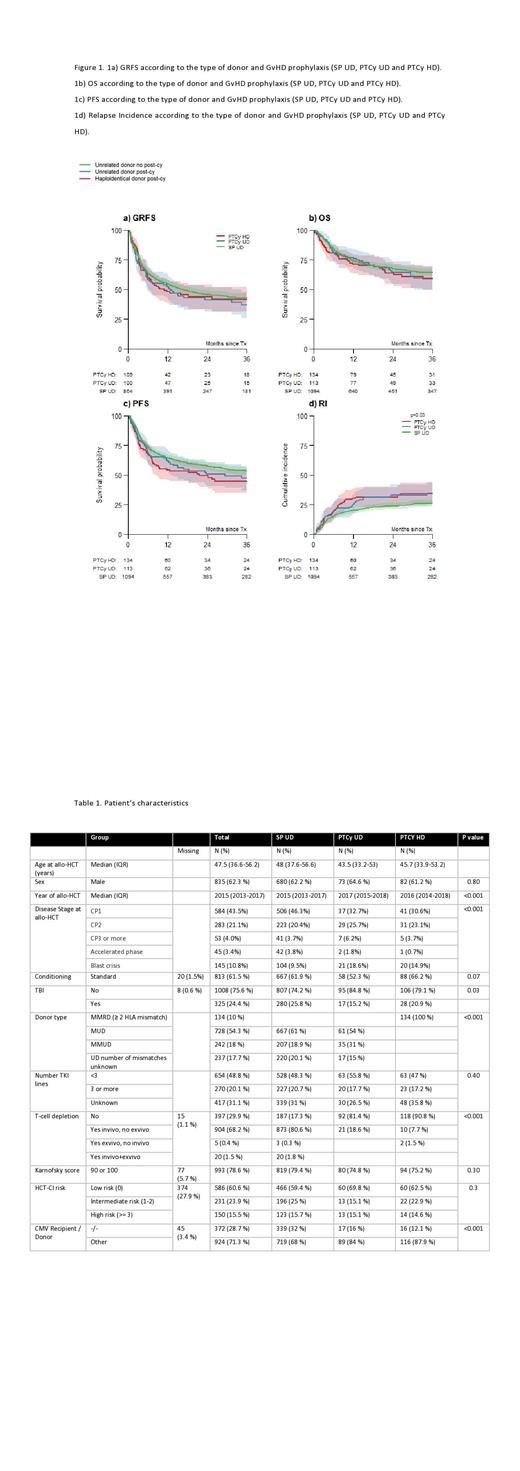
A total of 1,341 CML patients treated in EBMT centers were included. 1,094 patients received allo-HCT from SP UD, 113 from PTCy UD and 134 from PTCy HD. 61% and 54% of patients received an allo-HCT from matched UD in the SP UD and PTCy UD cohort, respectively. SP UD GvHD prophylaxis was calcineurin inhibitor-based in 88% of patients; some form of T-cell depletion was used in 82%, 19% and 9% in the SP UD, PTCy UD, and PTCy HD cohorts, respectively. Further transplant and patient details can be found in Table 1.
The median follow-up was 34.9 (IQR 13.2-61.2) months. One-year GRFS was comparable in the three cohorts: 56%, 53% and 48% in SP UD, PTCy UD, and PTCy HD, respectively (log-rank p=0.30). There was no significant difference in the cumulative incidence of aGvHD at D+100 (Gray's test p=0.10) and one-year cGvHD (p=0.40) among cohorts. Conversely, the 3-year relapse incidence (RI) was significantly lower in the SP UD cohort (26%), compared to the PTCy UD (35%) and PTCy HD (34%) cohorts (Gray's test p=0.03) and the 3-year progression-free survival (PFS) was 53% vs 47% vs 45% in the SP UD, PTCy UD and PTCy HD cohorts, respectively (log-rank p=0.05). 3-year NRM was similar among cohorts 20% vs 18% vs 21% in SP UD, PTCy UD and PTCy HD, respectively (Gray's test p=0.80) and the three cohorts had a similar 3-year OS (64%, 60% and 59% in SP UD, PTCy UD and PTCy UD, respectively) (p=0.50).
In total 902 patients (68%) had complete data. In MVA, higher age (HR per 10 year increase in age 1.24, 95% CI 1.06-1.45, p=0.007) and high HCT-CI (HR high vs. low 2.43, 1.59-3.71, p<0.001) were associated with higher NRM. Karnofsky score (KS) ≤ 80 (HR vs. 90 or 100 2.07, 1.55-2.78, p<0.001) and disease status other than CP1 (HR BC vs. CP1 2.11, 1.47-3.03, p<0.001) was associated with higher RI. Higher age, lower KS, high HCT-CI, and disease status other than CP1 were associated with lower OS and PFS. In MVA, we did not observe significant differences between the SP UD, PTCy UD, and PTCy HD cohorts.
Conclusion
Based on our results, PTCy resulted in comparable outcomes in UD and HD allo-HCT, when compared to SP UD allo-HCT, as GRFS, PFS, NRM and OS were similar. However, there was a trend to a lower RI and higher PFS in SP UD allo-HCT which may have reached significance in a larger cohort. Furthermore, the findings of this study confirm that PTCy HD allo-HCT in CML is a valid option for patients lacking a MSD or UD. In addition, disease stage pre-transplant remains a major prognostic factor with CP1 patients having better outcomes when compared to those with more advanced phases and ≥CP1 CML. Overall, our results provide information on the efficacy of PTCy in allo-HCT for CML, and show that PTCy UD and PTCy HD are feasible allo-HCT platforms for patients diagnosed with CML.
Ortí: BMS, Novartis, Incyte, Pfizer: Honoraria, Speakers Bureau. Kulagin: X4 Pharmaceuticals, Alexion, Apellis, Biocad: Research Funding; Novartis, Generium, Sanofi, Roche, Johnson & Johnson, Pfizer: Speakers Bureau. Apperley: Bristol Myers Squibb, Novartis: Honoraria, Speakers Bureau; Incyte, Pfizer: Honoraria, Research Funding, Speakers Bureau. Kröger: Novartis: Research Funding; Riemser: Honoraria, Research Funding; Sanofi: Honoraria; Neovii: Honoraria, Research Funding; Jazz: Honoraria, Research Funding; Gilead/Kite: Honoraria; Celgene: Honoraria, Research Funding; AOP Pharma: Honoraria. Bloor: Kite, a Gilead Company: Honoraria; Novartis: Honoraria. Angelucci: Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene BSM: Honoraria, Other: DMC; Blue Bird Bio: Honoraria, Membership on an entity's Board of Directors or advisory committees; Menarini-Stemline: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: steering commitee, Speakers Bureau; Vertex Pharmaceuticals: Honoraria, Other: DMC; Crispr therapeutics: Honoraria, Other: DMC; Glaxo: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees. Blaise: Jazz Pharmaceuticals: Honoraria. Lopez Corral: Gilead, Novartis: Consultancy, Honoraria. Anagnostopoulos: Abbvie: Other: clinical trials; Sanofi: Other: clinical trials ; Ocopeptides: Other: clinical trials ; GSK: Other: clinical trials; Incyte: Other: clinical trials ; Takeda: Other: clinical trials ; Amgen: Other: clinical trials ; Janssen: Other: clinical trials; novartis: Other: clinical trials; Celgene: Other: clinical trials; Roche: Other: clinical trials; Astellas: Other: clinical trials . Wrobel: Janssen: Honoraria, Speakers Bureau; Roche: Honoraria, Research Funding, Speakers Bureau; BMS: Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau; Takeda: Honoraria, Speakers Bureau; BeiGene: Honoraria, Speakers Bureau. Rigacci: Merck: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accomodations, Expenses; Gilead Science: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Menarini: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Hayden: Jansen, Takeda: Other: Travel, Accomodation, Expenses; Amgen: Honoraria. Chalandon: Incyte, BMS, Pfizer, Abbie, MSD, Roche, Novartis, Amgen: Other: Advisory Board; Incyte: Speakers Bureau; Incyte, BMS, Pfizer, Abbie, MSD, Roche, Novartis, Gilead, Amgen, Jazz, Astra Zenec: Other: Travel EXpenses, Accomodation. Yakoub-Agha: Jazz Pharmaceuticals: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal